男女共同参画学協会連絡会
支援企業による広告記事
- ソーラボジャパン株式会社
- CAD を使って光学装置を設計してみよう
- 「生物物理」2025年10月号
- ソーラボジャパン株式会社
- 次世代2光子顕微鏡―小型化がもたらす新たな可能性
- 「生物物理」2024年10月号
- ソーラボジャパン株式会社
- サイエンティフィックカメラと周辺機器の同期
- 「生物物理」2023年10月号
- ソーラボジャパン株式会社
- 顕微鏡のリノベーション ~ 顕微鏡ポートを活用した光学系の導入
- 「生物物理」2022年12月号
「Biophysics and Physicobiology」に Junichi Higo, Gert-Jan Bekker, Narutoshi Kamiya, Ikuo Fukuda, Yoshifumi Fukunishi による "Binding free-energy landscapes of small molecule binder and non-binder to FMN riboswitch: All-atom molecular dynamics" をJ-STAGEの早期公開版として掲載
2023年12月13日 学会誌
日本生物物理学会欧文誌[Biophysics and Physicobiology]に以下の論文が早期公開されました。
Junichi Higo, Gert-Jan Bekker, Narutoshi Kamiya, Ikuo Fukuda, Yoshifumi Fukunishi
"Binding free-energy landscapes of small molecule binder and non-binder to FMN riboswitch: All-atom molecular dynamics"
URL:https://doi.org/10.2142/biophysico.bppb-v20.0047
- Abstract
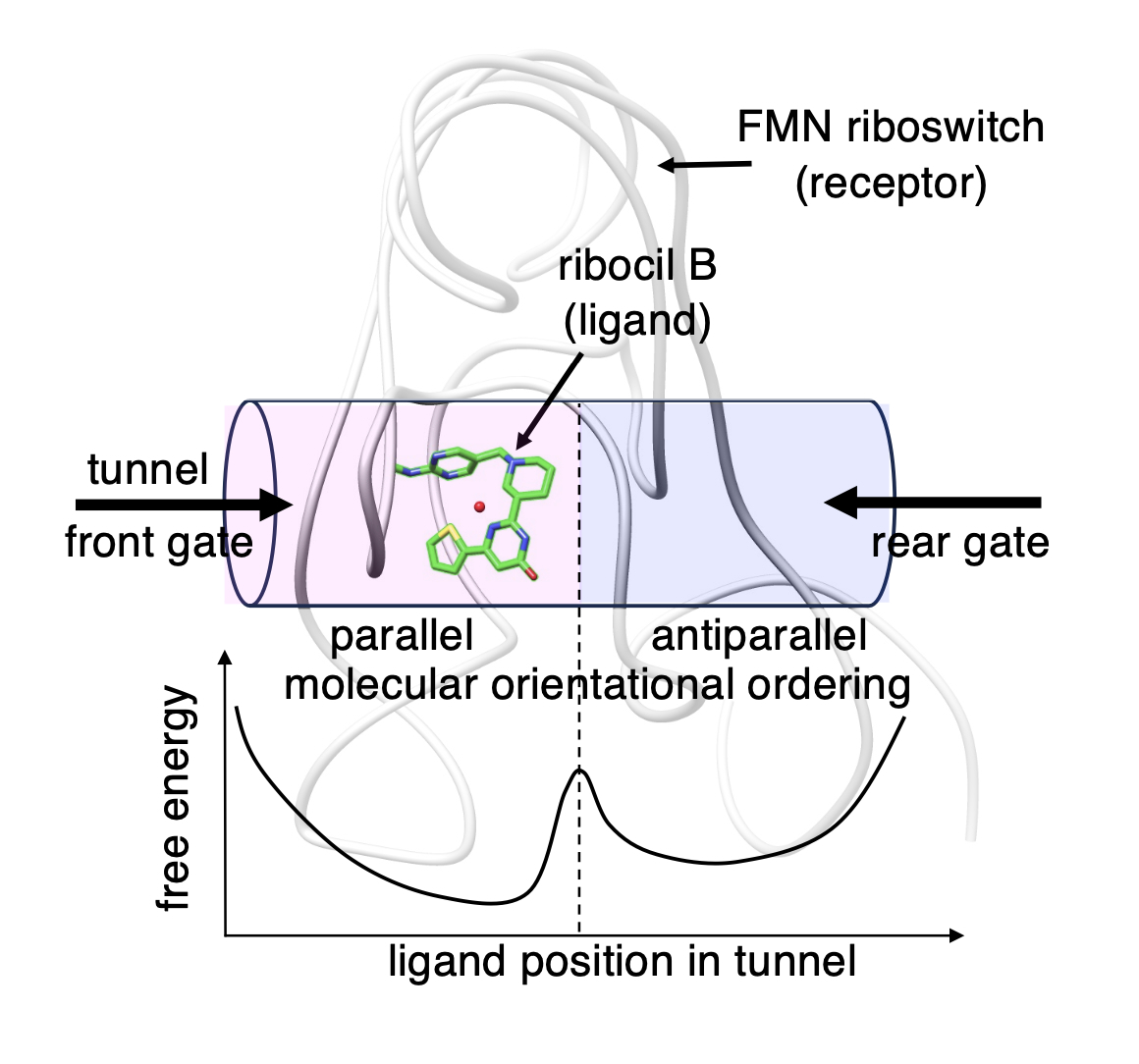
- A small and flexible molecule, ribocil A (non-binder) or B (binder), binds to the deep pocket of the aptamer domain of the FMN riboswitch, which is an RNA molecule. This binding was studied by mD-VcMD, which is a generalized-ensemble simulation method. Ribocil A and B are structurally similar because they are optical isomers to each other. In the initial conformation of simulation, the ligands and the aptamer were completely dissociated in explicit solvent. The aptamer–ribocil B binding was stronger than the aptamer–ribocil A binding, which agrees with experiments. The computed free-energy landscape for the aptamer–ribocil B binding was funnel-like, whereas that for the aptamer–ribocil A binding was rugged. When passing through the gate (named “front gate”) of the binding pocket, each ligand interacted with bases of the riboswitch by non-native π-π stackings, and the stackings restrained the ligand’s orientation to be advantageous to reach the binding site smoothly. When the ligands reached the binding site in the pocket, the non-native stackings were replaced by the native stackings. The ligand’s orientation restriction is discussed referring to a selection mechanism reported in an earlier work on a drug–GPCR interaction. The present simulation showed another pathway leading the ligands to the binding site. The gate (“rear gate”) for this pathway was located completely opposite to the front gate on the aptamer’s surface. However, the approach from the rear gate required overcoming a free-energy barrier regarding ligand’s rotation before reaching the binding site.
URL: https://doi.org/10.2142/biophysico.bppb-v20.0047