男女共同参画学協会連絡会
支援企業による広告記事
- ソーラボジャパン株式会社
- CAD を使って光学装置を設計してみよう
- 「生物物理」2025年10月号
- ソーラボジャパン株式会社
- 次世代2光子顕微鏡―小型化がもたらす新たな可能性
- 「生物物理」2024年10月号
- ソーラボジャパン株式会社
- サイエンティフィックカメラと周辺機器の同期
- 「生物物理」2023年10月号
- ソーラボジャパン株式会社
- 顕微鏡のリノベーション ~ 顕微鏡ポートを活用した光学系の導入
- 「生物物理」2022年12月号
「Biophysics and Physicobiology」に Yoshihiko Furuike, Eiki Yamashita, Shuji Akiyama による "Structure-function relationship of KaiC around dawn" をJ-STAGEの早期公開版として掲載
2023年12月16日 学会誌
日本生物物理学会欧文誌[Biophysics and Physicobiology]に以下の論文が早期公開されました。
Yoshihiko Furuike, Eiki Yamashita, Shuji Akiyama
"Structure-function relationship of KaiC around dawn"
URL:https://doi.org/10.2142/biophysico.bppb-v21.0001
- Abstract
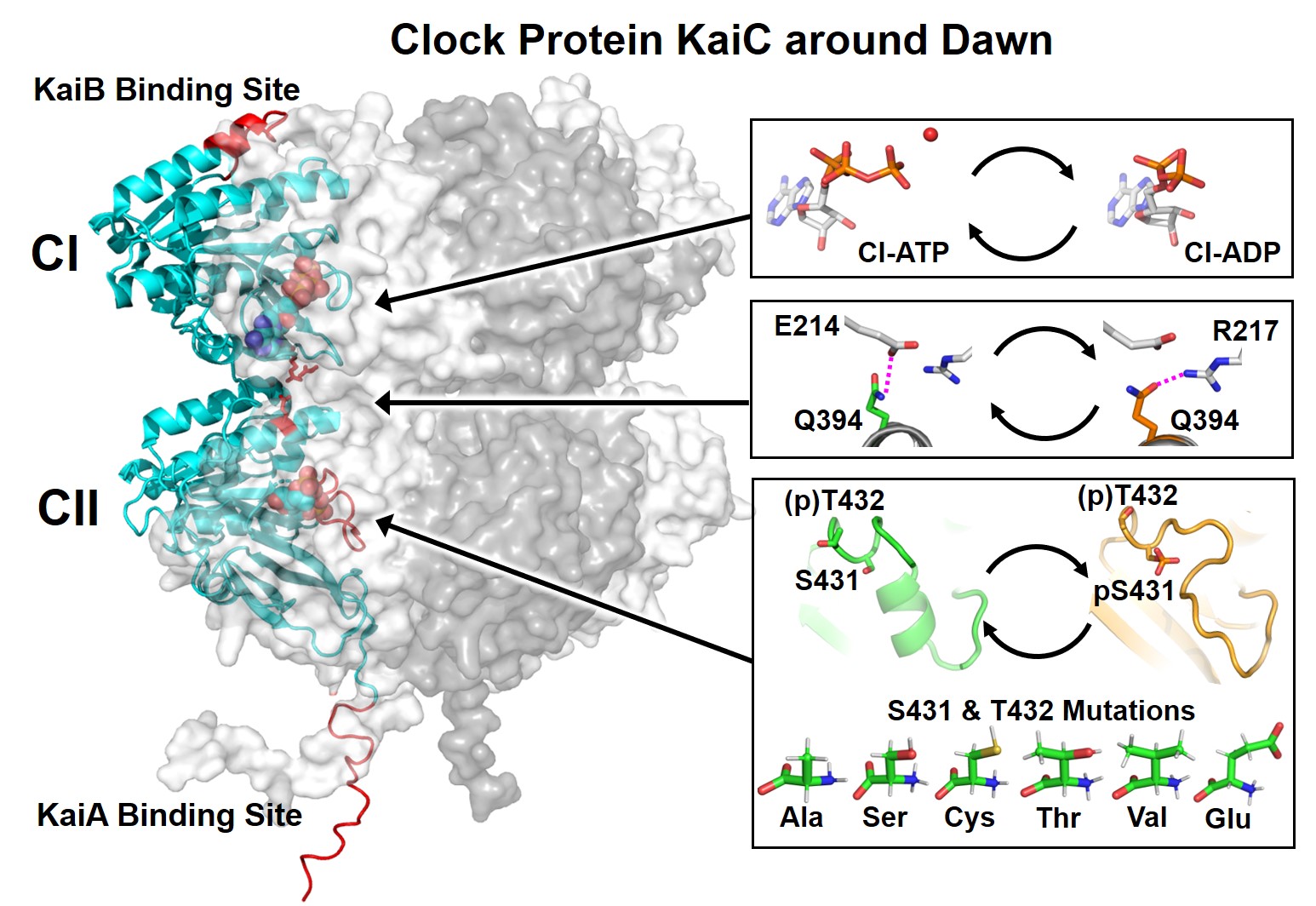
- KaiC is a multifunctional enzyme functioning as the core of the circadian clock system in cyanobacteria: its N-terminal domain has adenosine triphosphatase (ATPase) activity, and its C-terminal domain has autokinase and autophosphatase activities targeting own S431 and T432. The coordination of these multiple biochemical activities is the molecular basis for robust circadian rhythmicity. Therefore, much effort has been devoted to elucidating the cooperative relationship between the two domains. However, structural and functional relationships between the two domains remain unclear especially with respect to the dawn phase, at which KaiC relieves its nocturnal history through autodephosphorylation. In this study, we attempted to design a double mutation of S431 and T432 that can capture KaiC as a fully dephosphorylated form with minimal impacts on its structure and function, and investigated the cooperative relationship between the two domains in the night to morning phases from many perspectives. The results revealed that both domains cooperate at the dawn phase through salt bridges formed between the domains, thereby non-locally co-activating two events, ATPase de-inhibition and S431 dephosphorylation. Our further analysis using existing crystal structures of KaiC suggests that the states of both domains are not always in one-to-one correspondence at every phase of the circadian cycle, and their coupling is affected by the interactions with KaiA or adjacent subunits within a KaiC hexamer.
URL: https://doi.org/10.2142/biophysico.bppb-v21.0001