男女共同参画学協会連絡会
支援企業による広告記事
- ソーラボジャパン株式会社
- CAD を使って光学装置を設計してみよう
- 「生物物理」2025年10月号
- ソーラボジャパン株式会社
- 次世代2光子顕微鏡―小型化がもたらす新たな可能性
- 「生物物理」2024年10月号
- ソーラボジャパン株式会社
- サイエンティフィックカメラと周辺機器の同期
- 「生物物理」2023年10月号
- ソーラボジャパン株式会社
- 顕微鏡のリノベーション ~ 顕微鏡ポートを活用した光学系の導入
- 「生物物理」2022年12月号
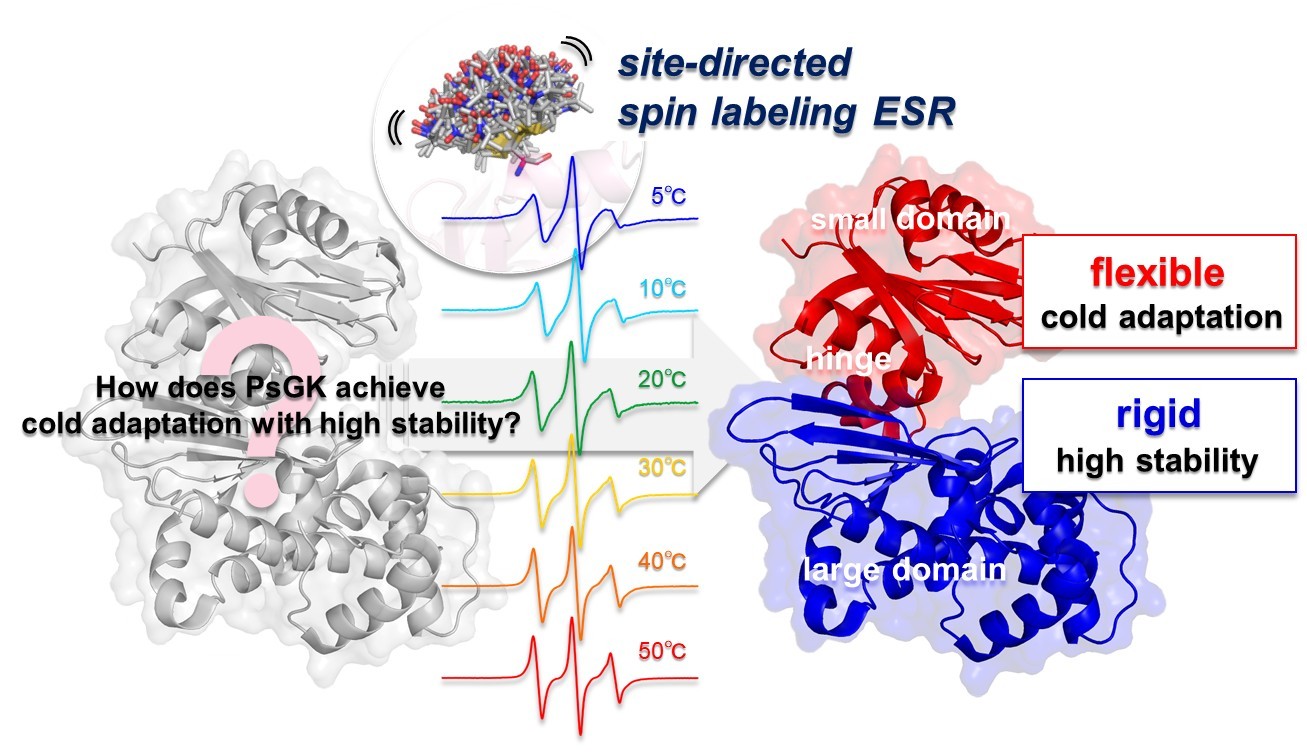
「Biophysics and Physicobiology」に Akane Yato, Masaki Horitani による "Mechanistic insights into a cold-adapted glucokinase with high thermal stability were revealed by site-directed spin labeling ESR" をJ-STAGEの早期公開版として掲載
2026年02月17日 学会誌
日本生物物理学会欧文誌[Biophysics and Physicobiology]に以下の論文が早期公開されました。
Akane Yato, Masaki Horitani
"Mechanistic insights into a cold-adapted glucokinase with high thermal stability were revealed by site-directed spin labeling ESR"
URL:https://doi.org/10.2142/biophysico.bppb-v23.0005
- Abstract
- The structural flexibility of enzymes plays an essential role in determining their catalytic efficiency and thermal stability. Cold-adapted enzymes are typically highly flexible, resulting in high catalytic activity but low stability. Glucokinase (GK) consists of the large substrates binding domain, small catalytic domain, and hinge region that undergoes conformational changes upon substrates binding. We recently reported that the psychrophilic GK from Pseudoalteromonas sp. AS-131 (PsGK) exhibits both high catalytic efficiency and remarkable thermal stability compared to the mesophilic GK from Escherichia coli (EcGK). We also found that a disulfide bond connecting the N- and C-termini in PsGK contributes to its unusual thermal stability. However, cold adaptation mechanism of cold-adapted PsGK has remained unclear. To clarify how PsGK acquires high activity, we utilized site-directed spin labeling electron spin resonance (SDSL-ESR) spectroscopy for PsGK and EcGK in the absence and presence of substrates in the wide range of temperatures. PsGK without substrates was more flexible than EcGK. Particularly, the small domain and hinge region of PsGK were highly flexible while its large domain was relatively rigid. In contrast, EcGK showed lower entire flexibility and did not exhibit domain dependent differences. When the substrates were bound, both enzymes became more rigid, but the small domain and hinge region of PsGK was still flexible whereas its large domain was considerably rigid. These results suggest that enhancing catalytic activity requires increasing flexibility only in proper sites rather than in the entire enzyme. These findings provide insight into how cold-adapted enzymes balance activity and stability.
URL: https://doi.org/10.2142/biophysico.bppb-v23.0005